Copper Electroplating : A Complete Guide
In industrial applications, inconsistent finishes and inadequate protection can result in costly rework and potential product failures. Copper electroplating offers a dependable and cost-effective solution, producing durable coatings that provide enhanced corrosion resistance and improved electrical conductivity. This electroplating technique allows industries to achieve consistent, high-quality finishes, thereby minimizing the risk of defects and ensuring the reliability of their products.
Copper electroplating is a method used to coat a metal surface with a thin layer of copper through an electrochemical process. By utilizing this technique, manufacturers can effectively tackle common challenges while ensuring operational efficiency and maintaining product integrity. This guide provides an overview of copper electroplating, detailing its techniques and exploring its applications across various industries.
Read on to discover everything you need to know about copper electroplating.
Copper Electroplating: A Brief Overview
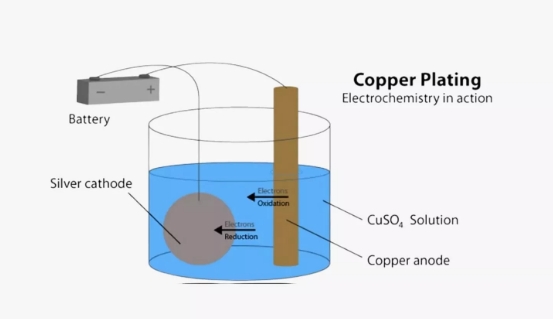
Copper electroplating is a method used to coat a metal surface with a thin layer of copper through an electrochemical process. This technique involves immersing the object to be plated into an electrolyte solution, usually containing copper sulfate, and applying an electric current. The object to be plated serves as the cathode (negative electrode), while a piece of pure copper acts as the anode (positive electrode). When current flows through the solution, copper ions are released from the anode and travel through the electrolyte, eventually depositing onto the cathode’s surface.
The result is a smooth, even layer of copper that enhances the object’s appearance, increases corrosion resistance. The process is widely used across industries such as electronics, automotive, and renewable energy, where durable metal finishes are essential.

When to Choose Electroplating with Copper
The choice of copper for electroplating hinges on the unique demands of the intended application. Copper’s exceptional conductivity and corrosion resistance render it highly suitable for electrical parts. Furthermore, its antibacterial qualities are highly esteemed in medical contexts. Most notably, its superior electrical conductivity stands out as a primary reason. This additional copper layer assists manufacturers in guaranteeing dependable electrical system performance, making it a prime choice for electronic components such as circuit boards that necessitate consistent and efficient current transmission.
Moreover, copper electroplating provides a warm and appealing aesthetic, making it ideal for architectural accents, jewelry, and household fixtures. Its efficient heat conduction capabilities also position it well for use in heat transfer system components. Its abundance and relatively economical pricing make it an affordable base coating option for industrial applications, without compromising on quality or performance.
Major Copper Electroplating Techniques
Below are the four primary techniques employed by industry professionals for copper plating metals.
Dual Damascene Plating
Dual Damascene Plating is an advanced technique utilized in the production of sophisticated microchips, playing a crucial role in improving both electrical performance and the miniaturization of devices. This process entails the formation of complex copper-filled structures within the circuitry of the microchip. By depositing copper into meticulously etched trenches and vias, Dual Damascene Plating facilitates efficient electrical signal flow through the chip, reducing resistance and enhancing speed.
This method is especially vital for contemporary electronic devices, where the demand for higher performance and smaller form factors is ever-increasing. The incorporation of copper-filled structures not only boosts conductivity but also enhances the overall reliability and durability of the microchip, making it more suitable for use in smartphones, tablets, and other advanced electronic devices.
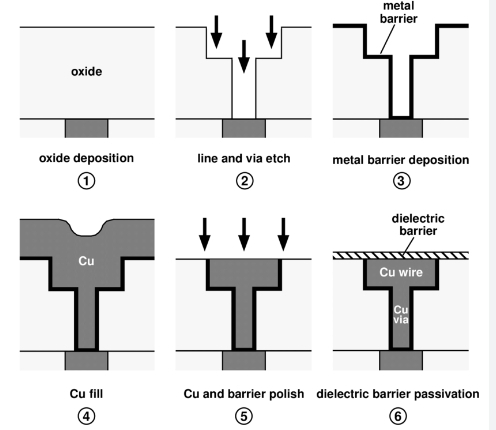
Image source: https://www.researchgate.net/figure/Simplified-dual-Damascene-process-flow-for-fabricating-Cu-interconnects_fig7_293488907
Through-Silicon Via (TSV) Plating
In the field of semiconductor manufacturing, Through-Silicon Via (TSV) Plating emerges as a groundbreaking process that significantly improves conductivity and interconnectivity. This technique involves connecting silicon wafers using copper-filled vias, thereby facilitating the construction of 3D integrated circuits that are vital for developing highly complex and compact electronic devices.
TSV plating enables the vertical stacking of multiple silicon wafers, effectively increasing the overall density and functionality of the integrated circuit. The copper-filled vias create a low-resistance pathway for electrical signals to traverse between different layers, ensuring rapid and efficient data processing and transmission. As such, TSV plating represents an essential step in the production of advanced semiconductors, paving the way for the innovation and manufacture of high-performance electronic products.
Copper Pillar Plating
Copper Pillar Plating is a crucial process in the domain of microelectronic packaging, as it ensures the reliability and performance of compact, high-speed electronic components. This technique involves the deposition of copper pillars on the surface of a microchip, acting as electrical connections between the chip and its packaging substrate. The copper pillars offer a robust and low-resistance pathway for electrical signals, facilitating the efficient and reliable operation of electronic components.
Furthermore, the compact size of the copper pillars enables increased density and a reduced footprint of the electronic package, making it particularly suitable for applications in smartphones, wearable devices, and other space-constrained environments. Consequently, Copper Pillar Plating is instrumental in advancing microelectronic packaging solutions, allowing for the creation of smaller, faster, and more efficient electronic devices.
Redistribution Layer (RDL) Plating
Redistribution Layer (RDL) Plating is a crucial process in advanced semiconductor manufacturing, optimizing connectivity within high-density packages. This technique involves applying a thin layer of conductive material, such as copper, onto the surface of a microchip, functioning as a redistribution layer for the chip’s input/output (I/O) pads. By rearranging and redistributing the I/O pads, RDL plating facilitates the development of more compact and efficient packaging solutions, which are essential for creating high-performance electronic devices.
This process increases density and minimizes interconnect length, thus enhancing signal integrity and reducing power consumption. Moreover, RDL plating provides greater flexibility and scalability in packaging design, allowing manufacturers to devise customized solutions that cater to specific customer requirements.
Different Types of Copper Electroplating Baths
Copper electroplating baths are classified based on their chemical composition and application suitability. The choice of bath type affects the quality, adhesion, and properties of the deposited copper layer. Below is a breakdown of the different types of copper electroplating baths and their characteristics.
1 . Typical Alkaline Copper Baths
Alkaline copper baths are commonly used in applications requiring thick, flexible copper coatings. These baths operate at higher pH levels, providing deposits that are both durable and ductile, making them ideal for parts that need to withstand mechanical stress. However, maintaining the precise pH balance is crucial to ensure uniformity and prevent impurities in the deposit.
Alkaline Cyanide Solutions
Historically popular, alkaline cyanide solutions produce high-quality copper coatings with excellent uniformity, especially for complex shapes. Cyanide-based baths provide exceptional control over the plating process, yielding a smooth, bright finish ideal for decorative and functional applications.
However, due to the toxicity and environmental impact of cyanide, its use is increasingly restricted, with disposal posing significant challenges. Today, many industries are moving away from cyanide solutions to prioritize worker safety and environmental compliance, though it remains a reliable option in limited, regulated settings where strict safety measures can be maintained.
Alkaline, Non-Cyanide Solutions
Non-cyanide alkaline copper baths have emerged as safer alternatives, delivering quality comparable to traditional cyanide methods without the hazardous byproducts. These solutions often rely on complexing agents to stabilize the copper ions, resulting in a reliable deposition process that is safer for both operators and the environment.
Non-cyanide baths are suitable for industries that prioritize safety and sustainability, such as electronics and automotive manufacturing. With advancements in non-toxic chemistry, these baths offer high-quality copper layers without compromising adhesion, thickness, or uniformity, making them a preferred choice for many modern electroplating applications.
Pyrophosphate Copper
Pyrophosphate copper baths provide a mildly alkaline environment, known for excellent throwing power, allowing copper to coat hard-to-reach areas. This makes pyrophosphate baths particularly valuable for plating complex electronic components with precise geometries.
Additionally, pyrophosphate baths operate at lower toxicity levels than cyanide-based options, making them safer for operators. These baths are often chosen for printed circuit boards and electronic connectors.

2 . Typical Acid Copper Baths
Acid copper baths are highly popular in industrial electroplating due to their efficiency, reliability, and cost-effectiveness. These baths use acidic copper sulfate solutions, which allow for high-speed copper deposition and are particularly well-suited for large-scale manufacturing.
Copper Sulfate Baths
Copper sulfate baths are among the most widely used types of acid copper baths, known for their affordability, reliability, and high plating speed. These baths work well with a range of substrates, allowing for consistent, thick copper layers that are ideal for industrial applications. The simplicity and high efficiency of copper sulfate solutions make them perfect for large-scale production, such as in printed circuit boards and electroforming. By carefully controlling factors like pH, temperature, and current density, manufacturers can achieve precise, uniform coatings that enhance both the appearance and function of plated parts.
Copper Fluoborate Baths
Copper fluoborate baths are chosen for applications that require rapid copper deposition and thicker layers. With fluoborate solutions, high deposition rates can be achieved, making these baths suitable for industries requiring fast turnaround and large-scale copper plating. Copper fluoborate solutions are highly conductive, allowing for thicker, even layers that are especially useful in heavy-duty applications where the copper layer will experience significant wear.
3 . Electroless Copper Plating Bath
Electroless copper plating is a non-electric plating method that enables copper deposition on non-metallic surfaces like plastics and ceramics. Unlike traditional electroplating, electroless plating relies on a chemical reaction to reduce copper ions onto the substrate, forming a uniform layer. This technique is often used as a preparatory layer for further electroplating or as a way to impart conductive properties to non-metallic components.
Electroplating Copper on Different Metals
| Metal | Plating Process & Description |
|---|---|
| Aluminum | Achieving a copper layer on aluminum requires a zincate pretreatment to remove the oxide layer naturally formed on the surface. This prepares the surface for copper deposition. |
| Steel | Copper is plated onto steel to enhance conductivity and provide a protective barrier against corrosion. This improves electrical conductivity and protects against environmental factors. |
| Nickel | Copper is directly plated onto nickel for applications demanding high wear resistance. This creates a durable surface layer that enhances performance in friction and wear conditions. |
| Brass | Copper electroplating on brass enhances its durability, resistance to corrosion, wear, and tarnishing, and improves both functional and decorative applications. |
| Zinc | Copper plating on zinc requires specialized processes to prevent galvanic corrosion, which occurs when two dissimilar metals come into contact with an electrolyte, accelerating corrosion. |

How to Electroplate Copper?
Copper electroplating is a widely used process that involves depositing a layer of copper onto a metal object using an electrolyte solution and electrical current. This process improves conductivity, corrosion resistance, and aesthetics. Below is a step-by-step guide on how to electroplate copper effectively.
1. Prepare the Workpiece:
Before starting the electroplating process, it’s crucial to prepare the metal object you want to plate. This involves cleaning the surface thoroughly to remove any dirt, grease, or oxides that could interfere with the copper plating. Use appropriate cleaning agents and techniques to ensure the object is completely clean and ready for the electroplating process. A clean surface will help ensure a smooth, even layer of copper is deposited.
2. Set Up the Electrolyte Solution:
The electrolyte solution is the key component in the electroplating process. It contains copper ions that will be deposited onto the workpiece during the plating process. A commonly used electrolyte solution for copper electroplating is copper sulfate. Mix the solution to the appropriate concentration and ensure it’s well-mixed before starting the process. The concentration of the solution will affect the rate and quality of the plating, so it’s important to follow the recipe carefully.
3. Connect the Workpiece as the Cathode:
Once the electrolyte solution is ready, immerse the cleaned workpiece in the solution. Connect the workpiece to the negative terminal (cathode) of a power source. This setup will ensure that copper ions in the solution migrate towards the workpiece during the plating process. Make sure the connection is secure and that the workpiece is properly immersed in the solution to avoid any plating issues.
4. Set Up the Anode:
The anode is another essential component in the electroplating process. It’s a piece of copper metal that will donate copper ions to the solution during the plating process. Place the copper anode in the electrolyte solution and connect it to the positive terminal (anode) of the power source. The anode should be positioned so that it’s fully immersed in the solution and can easily donate copper ions to the plating process.
5. Apply an Electrical Current:
With the workpiece and anode properly connected and immersed in the electrolyte solution, it’s time to apply an electrical current. Turn on the power source and allow the current to flow through the circuit. This will cause copper ions in the solution to migrate towards the cathode (workpiece) and deposit on its surface. Monitor the current and voltage to ensure they’re within the appropriate range for the desired plating rate and quality.
6. Plate the Copper:
Allow the electroplating process to continue for the desired duration. The length of time will depend on the thickness of copper you want to deposit on the workpiece. During this time, copper ions will continue to migrate towards the cathode and deposit on the surface of the workpiece. Monitor the process carefully to ensure uniform plating and to avoid any issues such as overplating or uneven deposition.
7. Remove and Clean the Workpiece:
Once the desired thickness of copper has been deposited on the workpiece, it’s time to remove it from the electrolyte solution. Carefully lift the workpiece out of the solution and rinse it with water to remove any residual solution. Dry the workpiece thoroughly to prevent any corrosion or tarnishing.
The Benefits of Copper Electroplating
Copper electroplating offers numerous advantages, making it a widely used technique in various industries. Its unique properties, such as flexibility, corrosion resistance, conductivity, and antibacterial qualities, provide significant functional and economic benefits. The table below highlights the key benefits of copper electroplating and their applications.
| Benefit | Description | Applications |
|---|---|---|
| Flexibility and Durability | Copper plating enhances structural integrity, improving resistance to wear, impact, and deformation. It is particularly beneficial for high-stress applications where durability is essential. | Automotive, aerospace, industrial machinery |
| Excellent Corrosion Protection | Copper forms a protective barrier against rust, oxidation, and environmental factors, extending the lifespan of metal components. | Marine equipment, outdoor structures, industrial machinery |
| Great Conductivity | Copper’s high electrical conductivity ensures efficient energy transfer, making it ideal for electronic and electrical applications. | Circuit boards, connectors, wiring, electrical components |
| Excellent Anti-Bacterial Qualities | Copper naturally eliminates bacteria and pathogens, making it suitable for sanitary environments where hygiene is critical. | Medical devices, food processing equipment, public facilities |
| Cost-Effectiveness | Copper is an affordable electroplating material that offers high-quality results at a lower cost compared to precious metals. | Large-scale manufacturing, budget-conscious industries, decorative applications |
Limitations of Copper Electroplating
While copper electroplating offers numerous advantages, it also comes with certain limitations that must be managed to ensure optimal performance. Issues such as adhesion difficulties, uneven coating, surface defects, and metal compatibility can impact the effectiveness and durability of the plating. The table below outlines these limitations and their possible solutions.
| Limitation | Description | Possible Solutions |
|---|---|---|
| Adhesion Issues | Achieving strong adhesion depends on the substrate material and plating bath type. Some metals, like aluminum, require special pretreatments to ensure proper bonding. Poor adhesion can cause flaking or peeling, reducing plating durability. | Proper surface preparation, using adhesion-promoting treatments, and maintaining bath composition can help enhance adhesion. |
| Uneven Coating | Complex shapes may experience inconsistent plating thickness due to uneven current distribution. This can lead to functional and aesthetic issues. | Adjusting bath agitation, using special plating equipment, and optimizing electrode positioning can improve coating uniformity. |
| Surface Defects | Impurities, air bubbles, and chemical imbalances in the electrolyte solution can cause defects like pits, roughness, or nodules, affecting the final product. | Regular monitoring, filtration of the electrolyte solution, and process control help maintain plating quality and minimize surface defects. |
| Compatibility with Other Metals | Copper can cause galvanic corrosion when in contact with metals like zinc or aluminum, leading to premature degradation in mixed-metal assemblies. | Using barrier coatings, insulation layers, or selecting compatible plating methods can help prevent galvanic reactions and ensure long-term performance. |
The 5 Main Applications of Copper Electroplating
Copper electroplating is a widely used process that enhances the properties of various materials by coating them with a thin layer of copper. This technique is valued for its excellent conductivity, corrosion resistance, and aesthetic appeal, making it essential in several industries. Below are the five primary applications of copper electroplating and how they contribute to different fields.
Electrical Wiring
Copper electroplating is widely used in electrical wiring due to its excellent conductivity and resistance to oxidation. Copper-plated wires facilitate efficient energy transfer, making them ideal for high-performance systems in telecommunications, data centers, and power transmission. The conductivity of copper helps reduce energy loss and ensures stable current flow, which is essential for sensitive equipment.
Electronics
In electronic components, copper electroplating enhances both durability and connectivity. Copper plating on circuit boards and connectors helps create a reliable conductive layer, ensuring stable connections in circuits. This conductivity is vital in devices where low resistance and high performance are required, such as smartphones, computers, and advanced medical equipment.

Automotive
Copper-plated parts in automotive applications provide essential benefits like corrosion resistance, conductivity, and enhanced durability. Components such as connectors, terminals, and wiring in vehicles benefit from copper’s anti-corrosive properties, especially in high-humidity environments.
Copper’s excellent conductivity also supports efficient power transfer within the electrical systems of modern vehicles. This ensures reliable performance in engine controls, sensors, and safety systems, making copper electroplating a valuable process in manufacturing durable and high-performing automotive components.
Renewable Energy
Copper electroplating is significant in renewable energy technologies, supporting efficient energy transfer in solar panels and wind turbines. Copper-plated components in these systems, such as connectors and wiring, enable efficient transmission of electricity generated from renewable sources.
Decorative Applications
Copper electroplating provides aesthetic appeal in decorative applications such as jewelry, home decor, and architectural features. Copper’s warm, lustrous appearance makes it a popular choice for creating visually appealing finishes that also resist tarnishing.
Get Aesthetic Metal Parts With VMT’s Finishing Services
VMT offers a variety of metal fabrication services, including CNC machining, sheet metal fabrication, die casting, and professional surface treatment processes to ensure that parts meet requirements in performance and appearance. Copper plating is one of our specialty services that can add beauty and functionality to metal parts. We are committed to providing high-quality copper plating services for industrial and decorative fields, creating parts that are both practical and durable.
We attach great importance to quality control to ensure that the products delivered meet consistent and precise standards every time. To this end, we have obtained ISO 9001 quality management system certification and passed SGS on-site audits. In addition, our service team is online 24 hours a day and can respond quickly and provide quotes within 2 hours to meet your needs.

In Conclusion
Copper electroplating is a widely used surface treatment method in various industries, which can provide durability, conductivity and corrosion resistance. Due to its versatility and effectiveness, copper electroplating has become a popular process choice to bring out the many benefits of copper while maintaining good mechanical properties. In addition, the copper coating protects the base metal from environmental and corrosion effects.
For copper electroplating or other manufacturing services, please feel free to contact VMT.
Frequently Asked Questions About Copper Electroplating
How do You Electroplate Copper?
Copper electroplating uses a copper sulfate solution and an electric current to coat an object with copper. The item to be plated connects to the cathode, while a pure copper piece connects to the anode. Passing a current through the solution causes copper ions to move to the object’s surface, creating a thin, even copper layer.
What Solution is Used for Copper Electroplating?
Copper sulfate serves as a prevalent solution in the realm of copper electroplating. When dissolved in water, it supplies copper ions that travel to the object undergoing plating. The inclusion of various additives within the solution plays a critical role in managing characteristics such as smoothness and thickness of the final product. Additionally, sulfuric acid may be incorporated to sustain an optimal pH level during the process. This carefully configured setup guarantees a stable electroplating process and yields high-quality results. Consequently, copper sulfate is recognized as an ideal choice for both industrial operations and smaller-scale applications.
What is the Best Voltage for Copper Electroplating?
A voltage range of 1 to 3 volts is generally considered optimal for copper electroplating, offering consistent outcomes while mitigating problems such as uneven coating or “burning.” Utilizing a lower voltage facilitates a slower and more controlled plating rate, resulting in a smooth and uniform copper layer. While adjustments can be implemented based on the specific electrolyte composition and the requirements of the project, adhering to this voltage range typically ensures high-quality electroplating with minimal defects.